Introduction
COVID-19 vaccination protects against a severe course of COVID-19. Nevertheless, individuals with hematologic malignancies (HM) remain at increased risk of COVID-19-related morbidity and mortality compared to the general population. However, these relative risks are unknown for individuals with specific hematologic cancers and treatments. We aimed to quantify the risk of severe COVID-19 among individuals with HM who had completed primary COVID-19 vaccination (i.e., two-dose generally, but three-dose in patients with a malignancy history). Therefore, we compared COVID-19 outcomes to those in individuals with no malignancy (NM) and individuals with solid malignancies (SM) who had completed primary COVID-19 vaccination. Thanks to unique centrally registered data on cancer care (Netherlands Cancer Registry, NCR), we were able to stratify risk for different hematologic malignancies.
Methods
We conducted a nationwide cohort study including all inhabitants of the Netherlands registered in the Personal Records Database on January 1, 2020, (n = 17 407 585) through linkage of data on cancer care, COVID-19 vaccination, SARS-CoV-2 testing, COVID-19-related hospitalization, and mortality. We used data from January 1, 2010, to December 31, 2022. We included individuals who completed primary COVID-19 vaccination at age 18 years or older and were SARS-CoV-2-infected more than two weeks later. Severe disease was defined as a SARS-CoV-2 infection resulting in hospital admission or death. We fitted generalized estimating equations and calculated adjusted odds ratios (aORs) and 95% confidence intervals (CIs) for the associations between an HM history and severe COVID-19, relative to a history with NM and SM. In addition, we calculated aORs for associations between malignancy history and COVID-19-related hospital admission, ICU admission, and mortality. Odds ratios were adjusted for age and sex as potential confounders. We conducted stratified analyses by type of hematologic malignancy and incidence period of SARS-CoV-2 infection. For technical reasons, we calculated aORs using a random sample of 350 000 SARS-CoV-2-infected individuals from the NM group.
Results
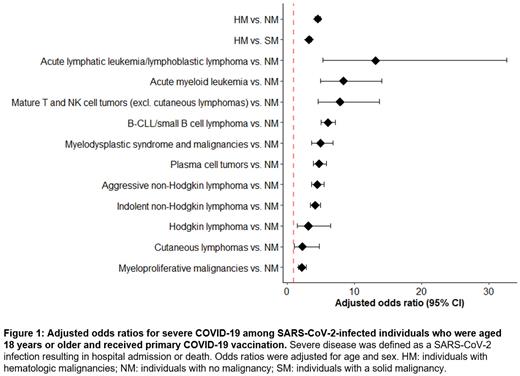
We included 8 210 individuals with HM, 350 000 individuals with NM, and 37 752 individuals with SM. We observed 8 552, 364 530, and 38 432 SARS-CoV-2 infections in individuals with HM, NM, and SM, respectively. Severe COVID-19 was recorded in 817, 2 834 and 1 412 cases, respectively. Individuals with HM were at higher risk of severe COVID-19 compared to individuals with NM and SM (aOR [95% CI] for HM vs. NM 4.6 [4.2-5.0]; for HM vs. SM 3.3 [3.0-3.7]) (Figure 1). Patients with HM were at increased risk for COVID-19-related hospitalization (aOR [95% CI] for HM vs. NM 4.6 [4.3-5.1]; for HM vs. SM 3.4 [3.1-3.7]), for COVID-19-related ICU admission (aOR [95% CI] for HM vs. NM 6.5 [5.0-8.4]; for HM vs. SM 6.3 [4.6-8.6]), and for COVID-19-related mortality (aOR [95% CI] for HM vs. NM 1.7 [0.9-3.0]; for HM vs. SM 1.9 [1.0-3.7]). Within the group of HM, those with a diagnosis of acute lymphatic leukemia/lymphoblastic lymphoma or acute myeloid leukemia were at the highest risk of severe COVID-19 compared to individuals with NM, while those with myeloproliferative malignancies or cutaneous lymphomas had the lowest risk (Figure 1). Stratified analyses by incidence period of SARS-CoV-2 infection demonstrated that the aORs for severe COVID-19 remained increased over time, comparing individuals with HM to those with NM. Adjustments for other confounders, such as socio-economic status, and stratified analyses by incidence year of malignancy, immunosuppressive treatment, and number of booster vaccinations are currently being performed.
Conclusions
Despite intensified COVID-19 vaccination, individuals with HM remain at increased risk for a severe course of COVID-19 compared to both individuals with NM and SM. However, the degree of vulnerability within the hematologic population depends on type of malignancy and this should be taken into account when offering additional booster vaccinations or scarce COVID-19 therapeutics, such as protease inhibitors or antibody-based therapy.
Disclosures
Zweegman:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding. Nijhof:Janssen: Honoraria, Speakers Bureau; Celgene/Bristol Myers Squibb: Honoraria, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal